Our Bodies are living laboratories. We are alive and breathing right now because of our complex nervous system in addition to the foods we eat and drink. This fuel we take in along with the oxygen from our lungs is “burned” in our cells, causing numerous chemical reactions to occur…until the products that are left are carbon dioxide (C02), water (H20) and an ash residue. This is similar to how our automobiles and fireplaces work, except their food is gasoline and firewood respectively. The question is…how does our ash residue measure up, and how does it affect our heath? The answer depends on whether this ash residue is acid or alkaline.
First of all let’s explore what it means to be acid or alkaline. What is pH?? No, we are not talking about how well your swimming pool is doing on a hot summer day. In the scientific world, pH stands for “potential of Hydrogen”. Instead, we’ll just say that, in your body, pH stands for your potential for Health.
pH is the value given to indicate the acidity or alkalinity of a substance. pH values are to acid and alkaline what temperature degrees are to heat and cold. It is merely a way of measuring the state of our ash. As we measure heat with degrees, ash residues that are acid, neutral or alkaline are measured by a “thermometer” called pH. The human body is composed of 78% water. Therefore, our bodies are made mostly of water, a vital medium which allows nutrients, oxygen and bio-chemicals to be transported throughout. This water based medium can be either acid or alkaline and is measured by the graduated pH scale. The ash residue produced by our human laboratory influences the pH of our body fluids and tissues.
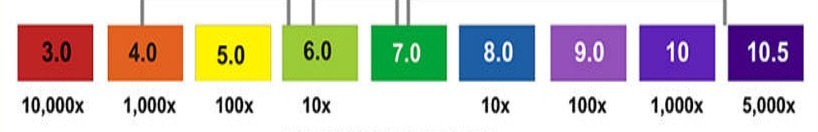
The pH scale of values runs from 0 to 14. At the low end, 0 indicates really strong, complete acidity. At the high end, 14 indicates really strong, complete alkalinity. In the middle, pH 7.0 indicates that the substance is neither acid nor alkaline – it’s neutral. Very few substances are completely neutral. Most substances test out on either side of neutral. For example, vinegar at pH 2.5, is a strong acid, and baking soda at pH 8.0, is slightly alkaline. When we talk about the pH of your body, we mean the pH of the fluids inside and outside your cells, your “internal environment”. pH is the potential for health of the fluids in and around your cells. The ideal pH for your internal environment is just above pH 7.0.
Why should I be concerned about my pH levels?
pH has a profound effect on health and disease. Imbalances in pH mean that the body has become too acidic or too alkaline for long periods of time which is not very well tolerated by the body. In fact, the body has regulatory mechanisms (breathing, circulation, digestion, hormonal production, etc.) that serve the purpose of managing and balancing pH levels. If the pH deviates too far to the acid side or too far to the alkaline side, cells become poisoned by their own toxins and die.
Life is all about balance. This is especially profound when speaking of body pH levels, which is the human body’s need to stay at a slightly alkaline pH. Levels of pH are measured on a scale from zero to 14, in which zero is very acidic, 7 is neutral, and 14 is very alkaline. Your body is over 70 percent water: For you to be healthy, your water content needs to have a healthy pH balance. Your body’s fluids are maintained at different pH levels to keep you healthy. To sustain these pH levels throughout your body, your cells and organs are constantly filtering what you eat. Everything works together to maintain your delicate pH balance.
First of all let’s explore what it means to be acid or alkaline. What is pH?? No, we are not talking about how well your swimming pool is doing on a hot summer day. In the scientific world, pH stands for “potential of Hydrogen”. Instead, we’ll just say that, in your body, pH stands for your potential for Health.
pH is the value given to indicate the acidity or alkalinity of a substance. pH values are to acid and alkaline what temperature degrees are to heat and cold. It is merely a way of measuring the state of our ash. As we measure heat with degrees, ash residues that are acid, neutral or alkaline are measured by a “thermometer” called pH. The human body is composed of 78% water. Therefore, our bodies are made mostly of water, a vital medium which allows nutrients, oxygen and bio-chemicals to be transported throughout. This water based medium can be either acid or alkaline and is measured by the graduated pH scale. The ash residue produced by our human laboratory influences the pH of our body fluids and tissues.
The pH scale of values runs from 0 to 14. At the low end, 0 indicates really strong, complete acidity. At the high end, 14 indicates really strong, complete alkalinity. In the middle, pH 7.0 indicates that the substance is neither acid nor alkaline – it’s neutral. Very few substances are completely neutral. Most substances test out on either side of neutral. For example, vinegar at pH 2.5, is a strong acid, and baking soda at pH 8.0, is slightly alkaline. When we talk about the pH of your body, we mean the pH of the fluids inside and outside your cells, your “internal environment”. pH is the potential for health of the fluids in and around your cells. The ideal pH for your internal environment is just above pH 7.0.
Why should I be concerned about my pH levels?
pH has a profound effect on health and disease. Imbalances in pH mean that the body has become too acidic or too alkaline for long periods of time which is not very well tolerated by the body. In fact, the body has regulatory mechanisms (breathing, circulation, digestion, hormonal production, etc.) that serve the purpose of managing and balancing pH levels. If the pH deviates too far to the acid side or too far to the alkaline side, cells become poisoned by their own toxins and die.
Life is all about balance. This is especially profound when speaking of body pH levels, which is the human body’s need to stay at a slightly alkaline pH. Levels of pH are measured on a scale from zero to 14, in which zero is very acidic, 7 is neutral, and 14 is very alkaline. Your body is over 70 percent water: For you to be healthy, your water content needs to have a healthy pH balance. Your body’s fluids are maintained at different pH levels to keep you healthy. To sustain these pH levels throughout your body, your cells and organs are constantly filtering what you eat. Everything works together to maintain your delicate pH balance.
Research states that the ideal level for drinking alkaline water is between pH8.5 – pH9.5 with an ORP of -250mv.
The Importance of Acid and Alkaline Balance for Health
Nothing does well in an overly acidic or alkaline pH medium. Similar to how acid rain can destroy a forest or how alkaline wastes can pollute a lake; an imbalanced pH can continuously corrode all body tissues, slowly eating into the 60,000 miles of our veins and arteries like rust eating into metal. This continued imbalance in pH will interrupt all cellular activities and functions, from the brain cell firing vital information through our nerves to our circulatory system pumping oxygen fuel throughout our body. Imbalanced pH interferes with our potential for true health and wellness!
Studies have shown that healthy people’s body fluids are slightly alkaline while the same fluids of those who are sick are acidic, ranging from slightly acidic to extremely acidic. In Dr. Mark Cochran’s book “The Secrets of pH Concerning Health and Disease” he states the body should remain in a slightly alkaline condition in order to avoid disease and premature aging.
Virtually all degenerative diseases including cancer, heart disease, arthritis, osteoporosis, kidney and gall stones, and tooth decay are associated with excess acidity in the body. While the body has a homeostatic mechanism that maintains a constant pH 7.4 in the blood, this mechanism works by depositing and withdrawing acid and alkaline minerals from other locations including the bones, soft tissues, body fluids and saliva. Therefore, the pH of these other tissues can fluctuate greatly. The pH of saliva offers a general window through which you can see the overall pH balance in your body.
Cancer cannot exist in an alkaline environment. All forms of arthritis are associated with excess acidity. Acid in the body dissolves both teeth and bones. It leaches calcium from bone resulting in osteopenia or osteoporosis. Whatever health situation you are faced with, you can monitor your progress toward a proper acid/alkaline balance by testing your saliva pH.
Monitoring your pH gives you a general indication of how well or how hard your body is working to survive your lifestyle. The results of your pH tests are indicators of how your body is responding to the foods you eat and to other stresses. The actual acid or alkaline level of your internal environment affects how your body functions.
The pH values you get when you test your urine or saliva are indications of how your body is functioning. When your body is at its pH best, it hums along smoothly and easily as a brand new Ferrari would hum riding along eh Pacific Coast Highway at sunset on a warm summer night. And when your body hums along smoothly and easily, your health and resultant quality of life has a good chance of doing the same. When your body is at less than its pH best, its hum may turn into an exhausted moan as it works overtime t survive. And when your body is exhausted, you are exhausted and you’re potential for a lower quality of life results.
The pH of your internal environment is a good indicator of how hard your body is working just to survive in today’s environment. When your pH values are too far below or too high above pH 7.0, your potential for health plummets.
Our efforts to establish and maintain good pH levels are often thwarted by 2 main things:
What we put into our bodies (eating, drinking, breathing, absorbing, etc
What we are not taking into our bodies that we should (nutrients, vitamins, minerals, water, etc.)
You simply compare the color of the paper to the color scale on the side of the package to reveal your pH. You may test your pH everyday!
Your salivary pH should stay in a range of 7.0– 7.5 for healthy body function. The best time to test your salivary pH is approximately 1 hour before a meal and 2 hours after a meal. If your urinary pH fluctuates between 6.0- 6.5 in the morning and between 6.5 – 7.0 in the evening, your body is functioning within a healthy range.
When saliva pH falls below 7.0 or urine pH falls below 6.0, your nutrients are not being absorbed, your body becomes toxic and your body’s cells are bathing in acid. Generally going unnoticed for years, this acid waste begins to silently corrode and eat away at your blood vessels, destroying cells and ultimately, entire organ function in your body. Without correcting your body pH, the damage becomes progressively worse,, and deadly over time. Fatigue is probably the major symptom or complaint of an overly acidic body.
The Importance of Acid and Alkaline Balance for Health
Nothing does well in an overly acidic or alkaline pH medium. Similar to how acid rain can destroy a forest or how alkaline wastes can pollute a lake; an imbalanced pH can continuously corrode all body tissues, slowly eating into the 60,000 miles of our veins and arteries like rust eating into metal. This continued imbalance in pH will interrupt all cellular activities and functions, from the brain cell firing vital information through our nerves to our circulatory system pumping oxygen fuel throughout our body. Imbalanced pH interferes with our potential for true health and wellness!
Studies have shown that healthy people’s body fluids are slightly alkaline while the same fluids of those who are sick are acidic, ranging from slightly acidic to extremely acidic. In Dr. Mark Cochran’s book “The Secrets of pH Concerning Health and Disease” he states the body should remain in a slightly alkaline condition in order to avoid disease and premature aging.
Virtually all degenerative diseases including cancer, heart disease, arthritis, osteoporosis, kidney and gall stones, and tooth decay are associated with excess acidity in the body. While the body has a homeostatic mechanism that maintains a constant pH 7.4 in the blood, this mechanism works by depositing and withdrawing acid and alkaline minerals from other locations including the bones, soft tissues, body fluids and saliva. Therefore, the pH of these other tissues can fluctuate greatly. The pH of saliva offers a general window through which you can see the overall pH balance in your body.
Cancer cannot exist in an alkaline environment. All forms of arthritis are associated with excess acidity. Acid in the body dissolves both teeth and bones. It leaches calcium from bone resulting in osteopenia or osteoporosis. Whatever health situation you are faced with, you can monitor your progress toward a proper acid/alkaline balance by testing your saliva pH.
Monitoring your pH gives you a general indication of how well or how hard your body is working to survive your lifestyle. The results of your pH tests are indicators of how your body is responding to the foods you eat and to other stresses. The actual acid or alkaline level of your internal environment affects how your body functions.
The pH values you get when you test your urine or saliva are indications of how your body is functioning. When your body is at its pH best, it hums along smoothly and easily as a brand new Ferrari would hum riding along eh Pacific Coast Highway at sunset on a warm summer night. And when your body hums along smoothly and easily, your health and resultant quality of life has a good chance of doing the same. When your body is at less than its pH best, its hum may turn into an exhausted moan as it works overtime t survive. And when your body is exhausted, you are exhausted and you’re potential for a lower quality of life results.
The pH of your internal environment is a good indicator of how hard your body is working just to survive in today’s environment. When your pH values are too far below or too high above pH 7.0, your potential for health plummets.
Our efforts to establish and maintain good pH levels are often thwarted by 2 main things:
What we put into our bodies (eating, drinking, breathing, absorbing, etc
What we are not taking into our bodies that we should (nutrients, vitamins, minerals, water, etc.)
- How Do I Test My pH?
You simply compare the color of the paper to the color scale on the side of the package to reveal your pH. You may test your pH everyday!
Your salivary pH should stay in a range of 7.0– 7.5 for healthy body function. The best time to test your salivary pH is approximately 1 hour before a meal and 2 hours after a meal. If your urinary pH fluctuates between 6.0- 6.5 in the morning and between 6.5 – 7.0 in the evening, your body is functioning within a healthy range.
When saliva pH falls below 7.0 or urine pH falls below 6.0, your nutrients are not being absorbed, your body becomes toxic and your body’s cells are bathing in acid. Generally going unnoticed for years, this acid waste begins to silently corrode and eat away at your blood vessels, destroying cells and ultimately, entire organ function in your body. Without correcting your body pH, the damage becomes progressively worse,, and deadly over time. Fatigue is probably the major symptom or complaint of an overly acidic body.